Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins
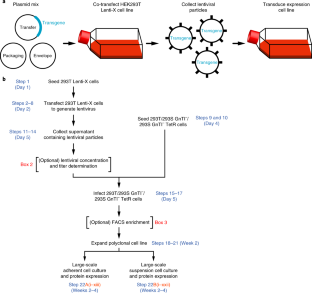
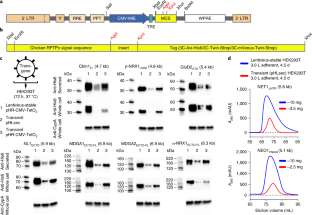
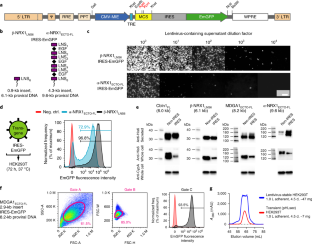
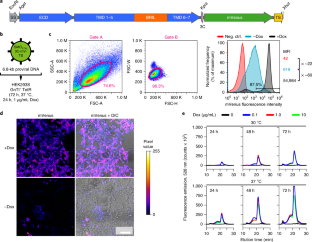
Structural, biochemical and biophysical studies of eukaryotic soluble and membrane proteins require their production in milligram quantities. Although large-scale protein expression strategies based on transient or stable transfection of mammalian cells are well established, they are associated with high consumable costs, limited transfection efficiency or long and tedious selection of clonal cell lines. Lentiviral transduction is an efficient method for the delivery of transgenes to mammalian cells and unifies the ease of use and speed of transient transfection with the robust expression of stable cell lines. In this protocol, we describe the design and step-by-step application of a lentiviral plasmid suite, termed pHR-CMV-TetO2, for the constitutive or inducible large-scale production of soluble and membrane proteins in HEK293 cell lines. Optional features include bicistronic co-expression of fluorescent marker proteins for enrichment of co-transduced cells using cell sorting and of biotin ligase for in vivo biotinylation. We demonstrate the efficacy of the method for a set of soluble proteins and for the G-protein-coupled receptor (GPCR) Smoothened (SMO). We further compare this method with baculovirus transduction of mammalian cells (BacMam), using the type-A γ-aminobutyric acid receptor (GABAAR) β3 homopentamer as a test case. The protocols described here are optimized for simplicity, speed and affordability; lead to a stable polyclonal cell line and milligram-scale amounts of protein in 3–4 weeks; and routinely achieve an approximately three- to tenfold improvement in protein production yield per cell as compared to transient transduction or transfection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
A nanobody-based strategy for rapid and scalable purification of human protein complexes
Article 16 November 2023

Development of a laboratory scalable process for enhancing lentivirus production by transient transfection of HEK293 adherent cultures
Article 27 April 2020

High-throughput 5′ UTR engineering for enhanced protein production in non-viral gene therapies
Article Open access 06 July 2021
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
- Aricescu, A. R. & Owens, R. J. Expression of recombinant glycoproteins in mammalian cells: towards an integrative approach to structural biology. Curr. Opin. Struct. Biol.23, 345–356 (2013). ArticleCASGoogle Scholar
- Chang, V. T. et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure15, 267–273 (2007). ArticleCASGoogle Scholar
- Aricescu, A. R., Lu, W. & Jones, E. Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr.62, 1243–1250 (2006). ArticleGoogle Scholar
- Chaudhary, S. et al. Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat. Protoc.7, 453–466 (2012). ArticleCASGoogle Scholar
- Naldini, L., Trono, D. & Verma, I. M. Lentiviral vectors, two decades later. Science353, 1101–1102 (2016). ArticleCASGoogle Scholar
- Benabdellah, K. et al. Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines. PLoS ONE6, e23734 (2011). ArticleCASGoogle Scholar
- De Groote, P. et al. Generation of a new Gateway-compatible inducible lentiviral vector platform allowing easy derivation of co-transduced cells. Biotechniques60, 252–259 (2016). ArticleGoogle Scholar
- Campeau, E. et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE4, e6529 (2009). ArticleGoogle Scholar
- Bandaranayake, A. D. et al. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Acids Res.39, e143 (2011). ArticleCASGoogle Scholar
- Chang, V. T. et al. Initiation of T cell signaling by CD45 segregation at ‘close contacts’. Nat. Immunol.17, 574–582 (2016). ArticleCASGoogle Scholar
- Chang, V. T., Spooner, R. A., Crispin, M. & Davis, S. J. Glycan remodeling with processing inhibitors and lectin-resistant eukaryotic cells. Methods Mol. Biol.1321, 307–322 (2015). ArticleGoogle Scholar
- Cronin, J., Zhang, X. Y. & Reiser, J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther.5, 387–398 (2005). ArticleCASGoogle Scholar
- Finkelshtein, D. et al. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA110, 7306–7311 (2013). ArticleCASGoogle Scholar
- Demaison, C. et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther.13, 803–813 (2002). ArticleCASGoogle Scholar
- Zufferey, R. et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol.15, 871–875 (1997). ArticleCASGoogle Scholar
- Lin, Y. C. et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun.5, 4767 (2014). ArticleCASGoogle Scholar
- Qin, J. Y. et al. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS ONE5, e10611 (2010). ArticleGoogle Scholar
- Reeves, P. J., Kim, J. M. & Khorana, H. G. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc. Natl. Acad. Sci. USA99, 13413–13418 (2002). ArticleCASGoogle Scholar
- Foecking, M. K. & Hofstetter, H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene45, 101–105 (1986). ArticleCASGoogle Scholar
- Gorman, C. M., Gies, D., McCray, G. & Huang, M. The human cytomegalovirus major immediate early promoter can be trans-activated by adenovirus early proteins. Virology171, 377–385 (1989). ArticleCASGoogle Scholar
- Donello, J. E., Loeb, J. E. & Hope, T. J. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol.72, 5085–5092 (1998). CASPubMedPubMed CentralGoogle Scholar
- Garcia-Nafria, J., Watson, J. F. & Greger, I. H. IVA cloning: a single-tube universal cloning system exploiting bacterial In Vivo Assembly. Sci. Rep.6, 27459 (2016). ArticleCASGoogle Scholar
- Schmidt, T. G. et al. Development of the Twin-Strep-tag and its application for purification of recombinant proteins from cell culture supernatants. Protein Expr. Purif.92, 54–61 (2013). ArticleCASGoogle Scholar
- Schmidt, T. G. & Skerra, A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat. Protoc.2, 1528–1535 (2007). ArticleCASGoogle Scholar
- Kumar, M., Keller, B., Makalou, N. & Sutton, R. E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther.12, 1893–1905 (2001). ArticleCASGoogle Scholar
- Knox, R. et al. A streamlined implementation of the glutamine synthetase-based protein expression system. BMC Biotechnol.13, 74 (2013). ArticleCASGoogle Scholar
- Mancia, F. et al. Optimization of protein production in mammalian cells with a coexpressed fluorescent marker. Structure12, 1355–1360 (2004). ArticleCASGoogle Scholar
- Oberbek, A., Matasci, M., Hacker, D. L. & Wurm, F. M. Generation of stable, high-producing CHO cell lines by lentiviral vector-mediated gene transfer in serum-free suspension culture. Biotechnol. Bioeng.108, 600–610 (2011). ArticleCASGoogle Scholar
- Shaner, N. C., Patterson, G. H. & Davidson, M. W. Advances in fluorescent protein technology. J. Cell Sci.120, 4247–4260 (2007). ArticleCASGoogle Scholar
- Lam, A. J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods9, 1005–1012 (2012). ArticleCASGoogle Scholar
- Goedhart, J. et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat. Commun.3, 751 (2012). ArticleGoogle Scholar
- Elegheert, J. et al. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science353, 295–299 (2016). ArticleCASGoogle Scholar
- Elegheert, J. et al. Structural mechanism for modulation of synaptic neuroligin-neurexin signaling by MDGA proteins. Neuron95, 896–913.e10 (2017). ArticleCASGoogle Scholar
- Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA99, 13419–13424 (2002). ArticleCASGoogle Scholar
- Yao, F. et al. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene Ther.9, 1939–1950 (1998). ArticleCASGoogle Scholar
- Brooks, A. R. et al. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med.6, 395–404 (2004). ArticleCASGoogle Scholar
- Hsu, C. C. et al. Targeted methylation of CMV and E1A viral promoters. Biochem. Biophys. Res. Commun.402, 228–234 (2010). ArticleCASGoogle Scholar
- He, J., Yang, Q. & Chang, L. J. Dynamic DNA methylation and histone modifications contribute to lentiviral transgene silencing in murine embryonic carcinoma cells. J. Virol.79, 13497–13508 (2005). ArticleCASGoogle Scholar
- Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE6, e18556 (2011). ArticleCASGoogle Scholar
- Li, Z. et al. Simple piggyBac transposon-based mammalian cell expression system for inducible protein production. Proc. Natl. Acad. Sci. USA110, 5004–5009 (2013). ArticleCASGoogle Scholar
- Matasci, M., Baldi, L., Hacker, D. L. & Wurm, F. M. The PiggyBac transposon enhances the frequency of CHO stable cell line generation and yields recombinant lines with superior productivity and stability. Biotechnol. Bioeng.108, 2141–2150 (2011). ArticleCASGoogle Scholar
- Boyce, F. M. & Bucher, N. L. Baculovirus-mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA93, 2348–2352 (1996). ArticleCASGoogle Scholar
- Dukkipati, A. et al. BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr. Purif.62, 160–170 (2008). ArticleCASGoogle Scholar
- Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc.9, 2574–2585 (2014). ArticleCASGoogle Scholar
- Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. Manipulation of subunit stoichiometry in heteromeric membrane proteins. Structure24, 797–805 (2016). ArticleCASGoogle Scholar
- Dull, T. et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol.72, 8463–8471 (1998). CASPubMedPubMed CentralGoogle Scholar
- Otto, E. et al. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum. Gene Ther.5, 567–575 (1994). ArticleCASGoogle Scholar
- Miyoshi, H. et al. Development of a self-inactivating lentivirus vector. J. Virol.72, 8150–8157 (1998). CASPubMedPubMed CentralGoogle Scholar
- Zufferey, R. et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol.72, 9873–9880 (1998). CASPubMedPubMed CentralGoogle Scholar
- Schermelleh, L., Spada, F. & Leonhardt, H. Visualization and measurement of DNA methyltransferase activity in living cells. Curr. Protoc. Cell Biol. Chapter 22, Unit 22.12 (2008).
- Hattori, M., Hibbs, R. E. & Gouaux, E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure20, 1293–1299 (2012). ArticleCASGoogle Scholar
- Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure14, 673–681 (2006). ArticleCASGoogle Scholar
- Davis, H. E., Rosinski, M., Morgan, J. R. & Yarmush, M. L. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys. J.86, 1234–1242 (2004). ArticleCASGoogle Scholar
- Backliwal, G. et al. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol. Bioeng.101, 182–189 (2008). ArticleCASGoogle Scholar
- Bell, C. H. et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science341, 77–80 (2013). ArticleCASGoogle Scholar
- Healey, E. G. et al. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat. Struct. Mol. Biol.22, 458–465 (2015). ArticleCASGoogle Scholar
- Byrne, E. F. X. et al. Structural basis of Smoothened regulation by its extracellular domains. Nature535, 517–522 (2016). ArticleCASGoogle Scholar
- Miller, P. S. & Aricescu, A. R. Crystal structure of a human GABAA receptor. Nature512, 270–275 (2014). ArticleCASGoogle Scholar
- Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics18, 529 (2017). ArticleGoogle Scholar
- Meissner, P. et al. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol. Bioeng.75, 197–203 (2001). ArticleCASGoogle Scholar
- Kutner, R. H., Zhang, X. Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc.4, 495–505 (2009). ArticleCASGoogle Scholar
- Telford, W. G. et al. Flow cytometry of fluorescent proteins. Methods57, 318–330 (2012). ArticleCASGoogle Scholar
- Robinson, J. P. & Roederer, M. History of science. Flow cytometry strikes gold. Science350, 739–740 (2015). ArticleCASGoogle Scholar
- Davies, D. Cell separations by flow cytometry. Methods Mol. Biol.878, 185–199 (2012). ArticleCASGoogle Scholar
- Andrell, J. et al. Generation of tetracycline-inducible mammalian cell lines by flow cytometry for improved overproduction of membrane proteins. Methods Mol. Biol.1432, 63–78 (2016). ArticleCASGoogle Scholar
Acknowledgements
We thank G. Davies and C. Green (University of Oxford) for assistance with flow cytometry, S. Padilla-Parra and L.A.J. Alvarez (University of Oxford) for assistance with microscopic imaging, D. Laverty (MRC-LMB) for the modified pEZT-BM vector, J. Watson (MRC-LMB) for advice on IVA cloning, and S. Ressl (Indiana University Bloomington) for comments on the manuscript. pMD2.G (Addgene plasmid no.12259) and psPAX2 (Addgene plasmid no. 12260) were a gift from D. Trono (École Polytechnique Fédérale de Lausanne (EPFL)). This work was supported by a Marie-Curie (FP7-328531) long-term postdoctoral fellowship (to J.E.); UK Medical Research Council grants MR/L009609/1 and MC_UP_1201/15 (to A.R.A.) and MR/L017776/1 (to C.S.); a UK Biotechnology and Biological Sciences Research Council grant (BB/M024709/1, to A.R.A.); Cancer Research UK grants C20724/A14414 (to C.S.) and C375/A10976 (to E.Y.J.); a European Research Council (ERC) grant (647278, to C.S.); and Wellcome Trust studentships (105247/Z/14/Z, to S.S., and 203726/Z/16/Z, to R.E.W.). The Wellcome Centre for Human Genetics is funded by Wellcome Trust Core Award 203852/Z/16/2.
Author information
- Jonathan Elegheert & Ester Behiels Present address: Interdisciplinary Institute for Neuroscience, CNRS UMR 5297, Bordeaux, France
- Eamon F. X. Byrne Present address: Department of Bioengineering, Stanford University, Stanford, CA, USA
- These authors contributed equally: Ester Behiels, Benjamin Bishop.
Authors and Affiliations
- Division of Structural Biology, Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK Jonathan Elegheert, Ester Behiels, Benjamin Bishop, Suzanne Scott, Rachel E. Woolley, Samuel C. Griffiths, Eamon F. X. Byrne, David I. Stuart, E. Yvonne Jones, Christian Siebold & A. Radu Aricescu
- MRC Laboratory of Molecular Biology, Cambridge Biomedical Campus, Cambridge, UK Suzanne Scott, Veronica T. Chang & A. Radu Aricescu
- Interdisciplinary Institute for Neuroscience, University of Bordeaux, Bordeaux, France Jonathan Elegheert & Ester Behiels
- Jonathan Elegheert










